Gunpowder made of sulfur, saltpeter, and charcoal
Table of Contents
109. Gunpowder made of sulfur, saltpeter, and charcoal; first on sulfur.
Gunpowder kindles fire most quickly. It made of:
- sulfur [fire-element]
- saltpeter [water-element]
- charcoal [earth-element]
Sulfur is as flammable as possible because it consists of particles of sharp juices so thinly and densely wrapped in the twigs of oily material that many branches between these twigs are open only to the fire-aether.
Hence, sulfur is even considered very hot for medicinal use.
110. Saltpeter
Saltpeter consists of oblong and rigid particles.
They are thicker at one end than at the other, different from common salt.
This can be deduced from the fact that when dissolved in water, it does not congeal into a square shape on its surface, as common salt does.
- Instead, it adheres to the bottom and sides of the vessel.
111. The Combination of sulfur and saltpeter
As for the size of the particles, it is to be thought that there is such a proportion between them that the sharp juices in sulfur, moved by the fire-aether, can easily shake off the air-aether globules from the gaps between the twigs of oily material.
At the same time, they agitate the saltpeter particles which are thicker than they are.
112. The motion of nitre particles
These particles of nitre are thicker in one part. They tend downward due to their gravity.
Consequently, their primary motion is in the sharper part, which is erect upward, as in B.
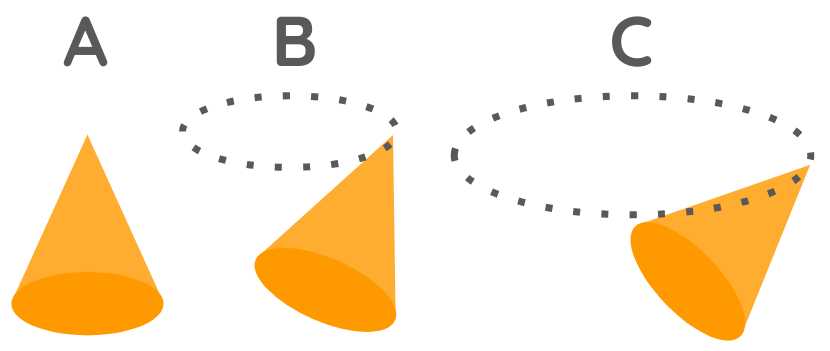
It forms a circular motion, initially small, as in C.
- But it immediately becomes larger, as in
D, unless something impedes it.
Meanwhile, particles of sulfur, moving very swiftly in all directions, reach other nitre particles in a very short time.
113. Why the flame of gunpowder expands greatly and moves upward.
Each of these nitre particles requires a significant amount of space to describe the circles of its motion.
The flame of this powder expands greatly. Furthermore, since these circles are described by the pointed end, the entire force of the flame tends upward.
Being very dry and fine, it can be ignited safely by hand.
114. Charcoal
Charcoal is mixed with sulfur and nitre.
From this mixture, it is moistened with some liquid to form small grains or pills which are then dried.
Many channels exist in charcoal from:
- the bodies that it was burned from
- the smoke that was created when those bodies were burned.
There are only 2 kinds of particles in it:
- The thicker
These form ashes on its own.
- The finer
This ignites easily because they were already moved by the force of fire. But these are intertwined with long and multiple branches, so they cannot be separated without some force.
This is evident from the fact that, while others go into smoke preceding their combustion, these last ones remain.
115. The grains of gunpowder and where its main force lies
Thus, sulfur and nitre easily enter the wide channels of charcoal.
They wrap around and constrict the branched particles of charcoal, especially when:
- moistened with some liquid
- compacted into grains or small pills, then dried.
The purpose of this is to ensure that the nitre particles all ignite in a single moment.
When the fire first touches the surface of any grain after being ignited elsewhere, it does not immediately inflame and dissolve it.
Instead, it takes some time to reach the inner parts of the grain from its surface.
There, with sulfur ignited first, it gradually agitates the particles of nitre so that, eventually, they, gaining strength and requiring more space for their circular motions, tear apart the bonds of charcoal and break the entire grain.
Even though this time is very short, if referred to hours or days, it is still considerable compared to the great speed with which the exploding grain spreads its flame throughout the nearby air.
For example, in a military device, a few grains of gunpowder ignited by a wick or another fuse immediately ignite others.
The flame erupting from them spreads through all the intervening spaces between the grains in the smallest moment of time.
While it may not penetrate the inner parts as quickly, because it touches many simultaneously, it causes many to ignite and expand simultaneously, thereby detonating the device with great force.
Thus, the resistance of charcoal greatly enhances the speed with which the particles of nitre burst into flame.
The distinction between grains is necessary to have sufficiently large channels around them, allowing the flame of the first ignited powder to freely reach many parts of the remaining powder.