The Substance of Conversion
Neutrons are the Substance of Convertibility


The Substance of Conversion
Section 1

The Substance of Conversion 1: High Energy Neutrinos
Section 2
The Strong CP Problem
Section 3b

Substance of Conversion 2: Neutrons
Section 3
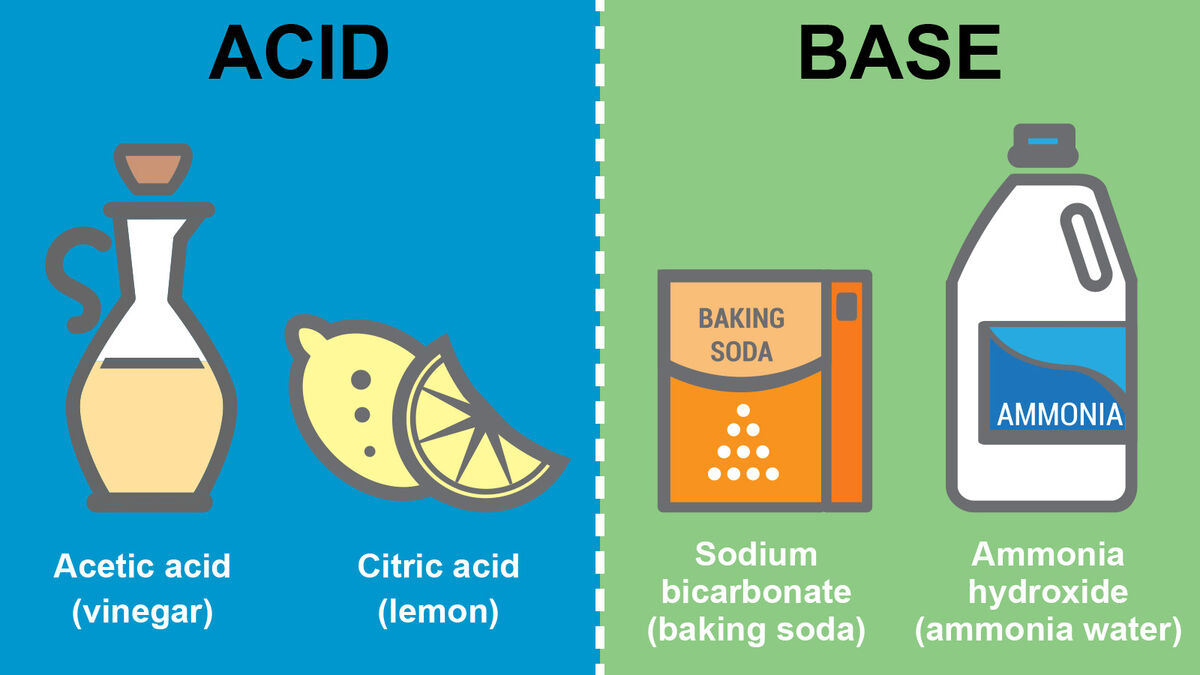
Substance of Conversion 3: Acids and Bases
Section 4